Intensive industrialized farming means thinking of nature as a factory.
If once agriculture treated the soil as a generous friend, intensive farming has gradually turned it into a chemical junkie. Pouring excess synthetic nitrogen fertilizers on fields has detrimental effects on the long-term vitality of the soil and on its capability to retain carbon.
This happens because the underground bacterial microflora (also responsible for actuating the natural nitrogen cycle) is brought out of balance by unnatural chemical fluxes, so that the soil gradually acidifies and loses its capacity for supporting crops. This process is technically referred to as soil degradation. “The depletion of the perpetual sources of that fertility”. It has been estimated that intensive agriculture accounts for 59% of man-made nitrous oxide emissions.
“The more fertilizer you use in agriculture, the more nitrous oxide you’ll get. These are small bacteria that convert nitrogen in the fertilizer into the by-product nitrous oxide” is stated, where the microbiota here is every reason to take good care of Earth’s ozone layer, because it takes good care of us.
“Without the ozone layer, no life could exist on Earth,” says Niels Larsen, the head of research at The Danish Climate Centre at the Danish Meteorological Institute. “It protects us against the sun’s ultraviolet rays, which would otherwise be so strong that we simply could not live here”.



Before the land is completely decimated there is of course, a window of time where the crop will yield, productively. But at what costs? It has been studied that the foods harvested from land treated with synthetic fertilizers are significantly lacking nutritional content. This effect is studied to increase over time, while the plants grow and grow, nutritional content is less and less present. Plants that grow in overly fertilized soil are deficient in: iron, zinc, carotene, vitamin C, copper and protein. Another negative effect has been reported by those who have direct contact with these materials, which can prove to be deadly or cause debilitating and long-term health complications.2 We at Green Peace Corps urge you to remember this the next time you go to pick up a bag of “fertilizer” or even potting “mix”. Synthetic fertilizers are sold under many brand names: Scotts, Miracle Gro (owned by Scotts), Shultz, Pennington, TruGreen, etc… Research the brand and the practices they follow, being aware of the plethora of lawsuits and settlements surrounding these brands is information you as the consumer deserve to be made aware of.
There have been reports that indicate negative health effects on the workers who handle fertilizers and work in spaces where the air is worsened by these products such as within a retail garden area. Let us examine why that is. In the U.S., there are three hyphenated numbers (for example 15-5-10) on the front label of fertilizer bags representing the percentage of each element by weight in the bag. The elements represented are N, P, and K. Nitrogen, Phosphorus, and Potassium. For example, the expression “15-5-10” means: 15% of the weight of the bag contains Nitrogen, 5% of the weight of the bag contains phosphorous, and 10% of the weight of the bag contains Potassium. Upon further reading on a bag, you will see an analysis of the types of chemical compounds used in order to reach these percentages. In other words, you will see a listing of the chemicals used to reach those percentages of 15-5-10. The synthetic chemicals used to reach these percentages of 15-5-10 can be different in different fertilizers. These components written on the bag are “guaranteed” to be in the bag by law. Some states have slightly different regulations. You will notice in the above example that 15-5-10 add up to a total of 30% of the weight of the bag. What is in the other 70% of the bag? It is not disclosed. It does not have to be revealed. It can be just about anything…including, and often, industrial waste. This mysterious 70% could contain clay, sand, sawdust, perlite, rice hulls, calcium carbonate (to dampen the detonation properties of the Nitrogen’s ammonium nitrate), corn cob grit, vermiculite, limestone, sludge, slag, or perhaps industrial waste. Your guess is as good as ours. Most people would be skeptical to believe that toxic residues from industry would be used to fill up most of a bag of fertilizer. Believe it. It is actually and unfortunately, widespread.



Up until this point we have largely been looking at the use of synthetic fertilizers and their effects, which are loved by many who put profits over the health and well-being of future generations. Petrochemical fertilizers which are another name for the synthetic products because they are produced using large quantities of petroleum and other fossil fuels. Some common examples include ammonium nitrate, super phosphate, and potassium sulfate. The nitrogen in petrochemical fertilizers may be composed of either water-soluble compounds or water-insoluble compounds. The percentage of each is always indicated on the label by the initials WSN or WIN. The water-soluble nitrogen is available immediately to plants — which is a huge advantage of synthetic fertilizers — and is the reason why there is such a pronounced, visible growth response in plants after fertilizer is applied. The water-insoluble nitrogen, also known as “slow-release” fertilizer, must be broken down in the soil before it is available to plants. This is also highly beneficial as an ongoing nutrient supply, meaning the fertilizer does not have to be applied as frequently.
There is little benefit to soil health from petrochemical fertilizers. Primarily because the nutrients are formulated to be absorbed directly by the plant, the soil becomes more of a growing medium rather than a source of fertility. If compared in terms of cost per pound of nitrogen and other nutrients, petrochemical fertilizers are generally much cheaper than their all-natural counterparts found on the shelves of garden centers. But there are many other cost-effective ways to provide natural fertilizers to plants that are not in the form of packaged fertilizer products — compost, manures, mulch, and soil-enriching “cover” crops, for example.
Volatile organic compounds (VOCs) are hydrocarbons emitted into the atmosphere by trees. They are part of the natural carbon cycle and may help to destroy ozone and methane when nitrous oxide concentration is normal. however, when nitrous oxide concentrations in the atmosphere are high (as they are today due to human-induced pollution), VOCs actually make ground level ozone and methane. Some have used this as an argument for deforestation, but it is not only more logical but also more cost-effective to stop polluting with nitrous oxide instead of cutting down forests.




Fertilizer is any substance used to add nutrients back into the soil to promote soil fertility and increase plant growth and crop yield. Nitrogen is known for promoting rapid, excessive green growth (much like steroids in a human). Nitrogen is the big player in commercial fertilizers. The appearance of rapid lush green growth occurs when applied. While this does drive sales of commercial synthetic fertilizers, all is not as simple as it may seem. The air is about 80% nitrogen. In nature, this is where the nitrogen nutrient originates. It can be brought into the soil by rain, other plants (such as legumes: alfalfa, clover, peas, etc.), other organisms (such as blue-green algae or microbes), or through the decay of other green plants, etc… These practices are integral to soil health and rely upon the cyclical nature of plant matter. These methods of fertilization take both time and labor to integrate but, the results are significant when we consider the vast health benefits, we, as consumers, ultimately receive, along with the long-term health benefits of the soil, which in turn increases the potential of a healthier future.
Unfortunately, consumers are conditioned to expect all fruits and vegetables to be available year-round and as a result unsustainable farming and gardening practices continue, but for how long can this continue? “Long-term use of synthetic fertilizers has a negative impact on soil structure, according to a study published recently in the Journal of Environmental Quality. Researchers at Kansas State University, who have been observing the effects of inorganic fertilizer use on soil properties during a 50-year study, found that while the application of inorganic sources of nitrogen and phosphorus may increase amounts of organic carbon, these gains may be offset by hidden costs such as decreased soil macro aggregates. Soil aggregate stability is a key indicator of soil structural quality because it affects how resistant soil is to erosion and how well water is able to move through the soil. The decreased soil aggregates could be due to ammonium ions in the synthetic fertilizer, which cause soil particles to separate rather than aggregate. This could offset any positive effects of increased soil organic carbon content, while increasing fertilizer runoff that causes pollution of waterways.”1 By damaging the structure of soil, we are creating a problem that may not have such a simple solution. The long-term effects will be extremely harmful to everyone’s way of life, the eventual destruction of the soil itself results in an uncertain foundation for all.

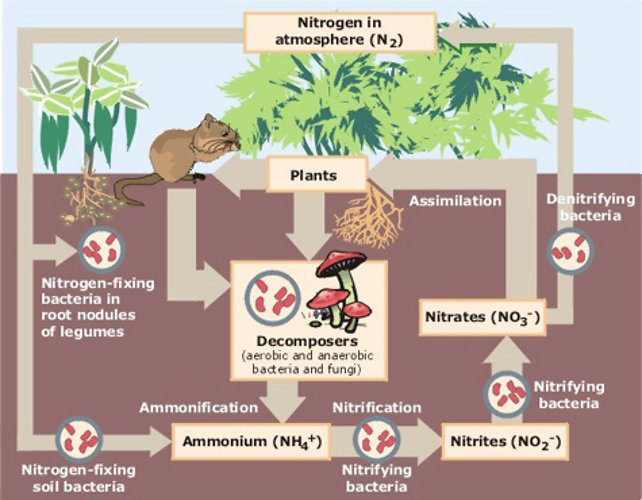
Another thing to for us to think about is that every 1% of organic matter in soils sequesters round 10 tons of carbon per acre. Therefore, synthetic fertilizers are partially responsible for the lack of the amount of necessary organic matter in commercially chemicalized soils which need nearly 5% organic matter or more for healthiest plant growth. This also signifies that commercial synthetically fertilized crops are carbon emitters and do not sequester carbon.
Commercial synthetic fertilizers boomed following World War ll as well as for the use of munitions and explosives during the war. Ammonium nitrate (NH3NO3): Ammonia, itself, is extremely toxic to humans. Easily having reactions if exposed to a variety of metals (i.e., iron, zinc, copper), acids, alkalis, solvents, oil, grease, etc. You will notice that bags of fertilizer are often plastic coated and sealed to keep contaminants out and gases in. Storage alone will give off ammonia, introduce heat to fertilizer and there will be further instability. Exposure to ammonium nitrate can cause a whole range of eye and skin irritations and inhalation exposure can result in a plethora more. The accumulation of ammonia in the body can quickly lead to death.
While the health of humans in contact with these chemicals is directly impacted, it is also, not isolated. There are about 50 billion microbes in a tablespoon of healthy soil. Many more near the roots of plants. Their primary job is to breakdown organic matter and to also feed plants. You could have every element in its proper proportion available in the soil, but without the “healthy” microbial action plants would not be able to utilize them. Synthetic chemical fertilizers inhibit, kill and alter this natural microbial activity which is so very important to healthy plants. In other words, synthetic fertilizers can dramatically diminish the nutritional value of foods. Since synthetic fertilizers also diminish the natural disease-fighting and pest-fighting mechanisms, our foods thus become laden with other chemicals (pesticides and herbicides).
“Fertilizer is good for the father and bad for the sons.”3
The biggest issue facing the use of chemical fertilizers is groundwater contamination. Nitrogen fertilizers break down into nitrates and travel easily through the soil as it is extremely water-soluble and can remain in groundwater for decades, the addition of more nitrogen over the years has an accumulative effect. One popular fertilizer, urea, produces ammonia emanation, contributes to acid rain, groundwater contamination and ozone depletion due to release of nitrous oxide by denitrification process. With its increased use and projections of future use, this problem may increase several times that in the coming decades. Groundwater contamination has been linked to a nearly endless list of destructive effects of human health but also environmental.
Nitrogen groundwater contamination also contributes to marine “dead zones”. The increase in the water-soluble nitrates creates an influx of plant-life, which eats up oxygen and starves out fish and crustaceans. This has an impact not only on the aquatic ecosystem, but on local societies who depend on food sourced from those areas. Perhaps one of the scariest effects of chemical fertilizers is something called methemoglobinemia in infants. The risk most often occurs when infants are given formula reconstituted with nitrate contaminated water. The condition causes a decrease in oxygen in the blood and results in a blue-grey skin color and can lead to coma or death. In ill-advised efforts to grow enough food to feed our citizens, we are simultaneously poisoning them.


In 2018, experts on a United Nations panel aimed at addressing the issue concluded that the world needs to cut the amount of nitrogen making its way into the environment in half to avoid disastrous consequences.
“In Iowa, we found that most counties, on average, were way over that point,” she said. “And it’s because nitrogen is sort of an insurance policy. Putting on more is not going to hurt anything on your farm . . . and it’s going to ensure that the crops grow.”
The number-one hotspot spans 61 corn- and soy-heavy counties in Illinois, Indiana, Missouri, and Wisconsin, and the next three are similar, in overlapping or neighboring states including Kansas, Nebraska, Iowa, and South Dakota. California’s largest hotspot unsurprisingly includes 21 counties that cover the Central Valley, America’s produce capital. And there are hotspots at the mouth of the Gulf of Mexico and surrounding the Chesapeake Bay.
Resourceful gardeners can generally find a way to increase the fertility of their soil without spending much money at all, while using synthetic fertilizers inevitably requires making a purchase. A purchase that ultimately undermines the long-term health of your plants, soil, water, and self. Is it worth the cost?
- The Organic Center, SEP 15, 2013, 50-year study on the impacts of inorganic fertilizer shows reduced soil structural stability, USDA NRCS South Dakota
- Health Effects of Synthetic Fertilizer (southlandorganics.com)
- Negative Effects of Chemical Fertilizers – AgriHunt
- The Carbon Farming Solution…book by Eric Toensmeier

